Water Softeners

Are you tired of hard water problems?
K and R Water Service has been serving Palm Beach County families for over 20 years. Our family owned and operated business is always available for your water needs, we understand what is means to have fresh, clean and great tasting water from your tap so that you can be worry free around the home.
More than 8 out of 10 American homes are affected by hard water—that is, water filled with dissolved minerals such as calcium and magnesium carbonate. While these minerals aren’t harmful for most people to ingest, hard water can cause a lot of inconveniences in everyday home life.
Are you ready to say goodbye to hard water problems?
What makes water hard?
Ground water dissolves rocks and minerals releasing calcium and magnesium ions that cause water to be hard. These dissolved ions give hard water its characteristics.
Problems caused by hard water:
Hard water interferes with all types of cleaning tasks. Cleaning problems arise when the cleaning agents do not fully remove dirt and grime. Over time, clothes washed in hard water may look dingy and feel harsh and scratchy. White clothing continually washed in hard water will gradually show a grayish tinge. Dishes and glassware washed in dishwashers using hard water may be spotted when dry. Hard water causes films on glass shower doors, walls and bathtubs. Hair washed in hard water may feel sticky and look dull. Regular soaps combine with dissolved calcium and magnesium to form soap curds or soap scum. Soap scum is difficult to remove from sinks and appliances.
When heated, calcium carbonate and magnesium carbonate are removed from the water and produce a scale buildup in the water heater. A large scale buildup slows the heating process and requires more energy to heat water. Water heaters with large accumulations of mineral buildup will have shorter life spans. Scale deposits also corrode and plug plumbing fixtures and accumulate in other appliances affecting their performance.
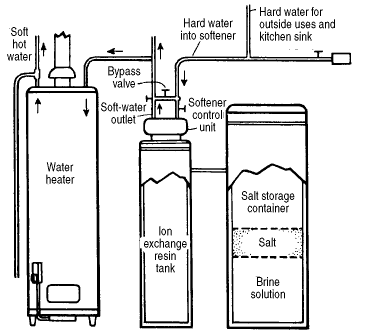
How do water softeners work?
A typical water-softening system removes calcium and magnesium ions from hard water and replaces them with sodium ions. Calcium and magnesium ions interfere with the action of household soaps and detergents, but sodium does not. The water-softening process thus helps detergents to more effectively remove dirt and oils from clothing and dishes. It also helps soaps to give a “slippery” feel to your skin when you wash. With soft water the soap and detergents rinse free leaving only your natural oils which makes your skin and hair feel slippery or silky. PureTap recommends that you reduce the amount of soap and detergents you use after installing a water softener.
The water softener unit is located in your household plumbing near the place where water enters the house so that it softens the water used for drinking and washing but not for irrigation. The unit contains several cubic feet of porous plastic resin covered with molecules that attract and bind to positive ions dissolved in the water. Normally, sodium positive ions coat the resin, but as water flows over the resin on its way to your sink or washer, the naturally occurring calcium and magnesium positive ions that exist in hard water stick to the resin. This releases sodium ions into the water in order to maintain a balance of electrical charge on the resin. Gradually, most of the sodium ions are released into the household water, and the resin becomes saturated with calcium and magnesium ions. Every few days, the unit must renew the resin by rinsing it with a concentrated solution of saltwater (sodium chloride), usually in the middle of the night. The high concentration of sodium ions in the salty water displaces the calcium and magnesium ions the resin, and the resin becomes once again covered with sodium ions. The salty rinse water, calcium and magnesium ions are flushed down the drain, and the system resumes normal operation. (Every so often it is necessary to add a bag of sodium chloride salt to the softener unit to prepare this salty rinse water.)
Each cubic foot of resin can effectively remove calcium and magnesium from about 3,200 gallons of hard water, which the Water Quality Association defines as 10 grains per gallon hardness. The process adds about 750 milligrams of sodium to each gallon of water, which the U.S. Food and Drug Administration considers to be in the “low sodium” range for commercially sold beverages. For people who are concerned about their overall intake of sodium, resins that instead release potassium into the water do exist, but the potassium chloride salt used to renew the resin every few days is more expensive than ordinary sodium chloride salt.
Ready to Enjoy Better, Softer Water?
Call now to learn more about how we can help you reduce the hard water problems in your home.
Phone 561-795-8675 TODAY!